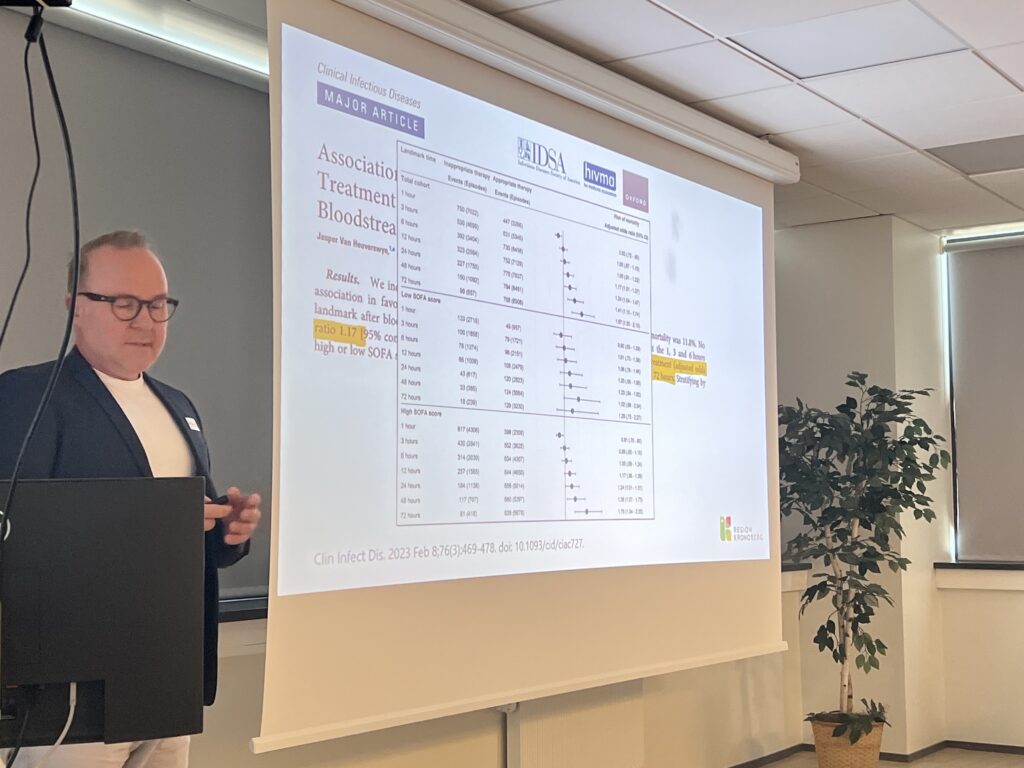
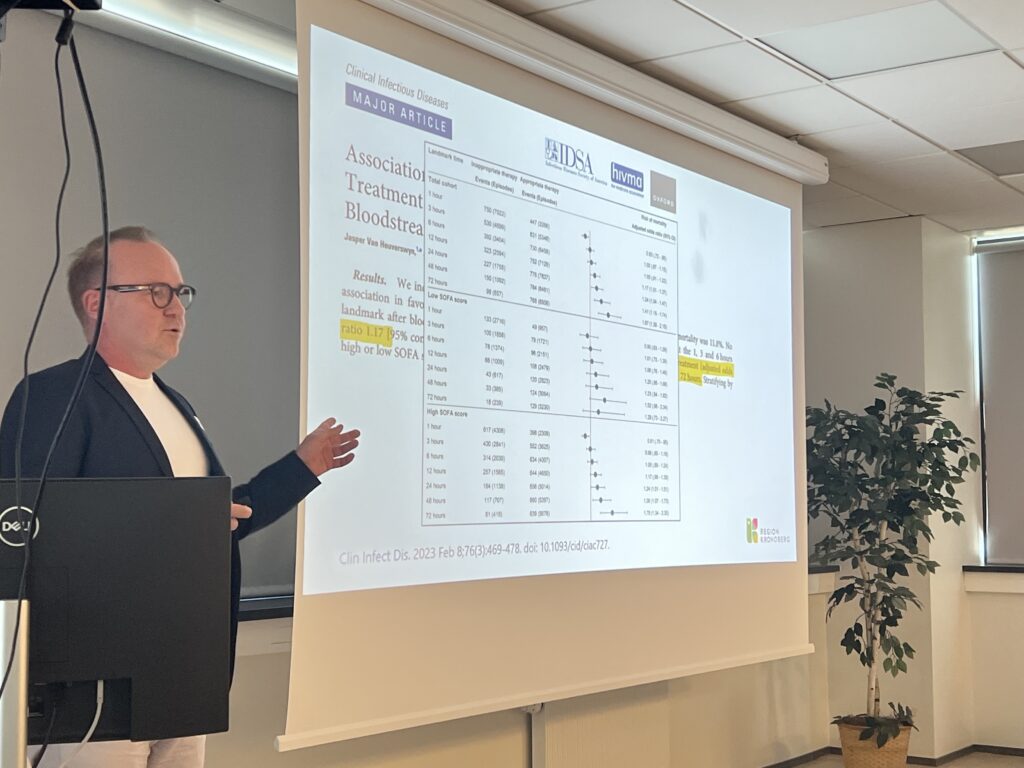
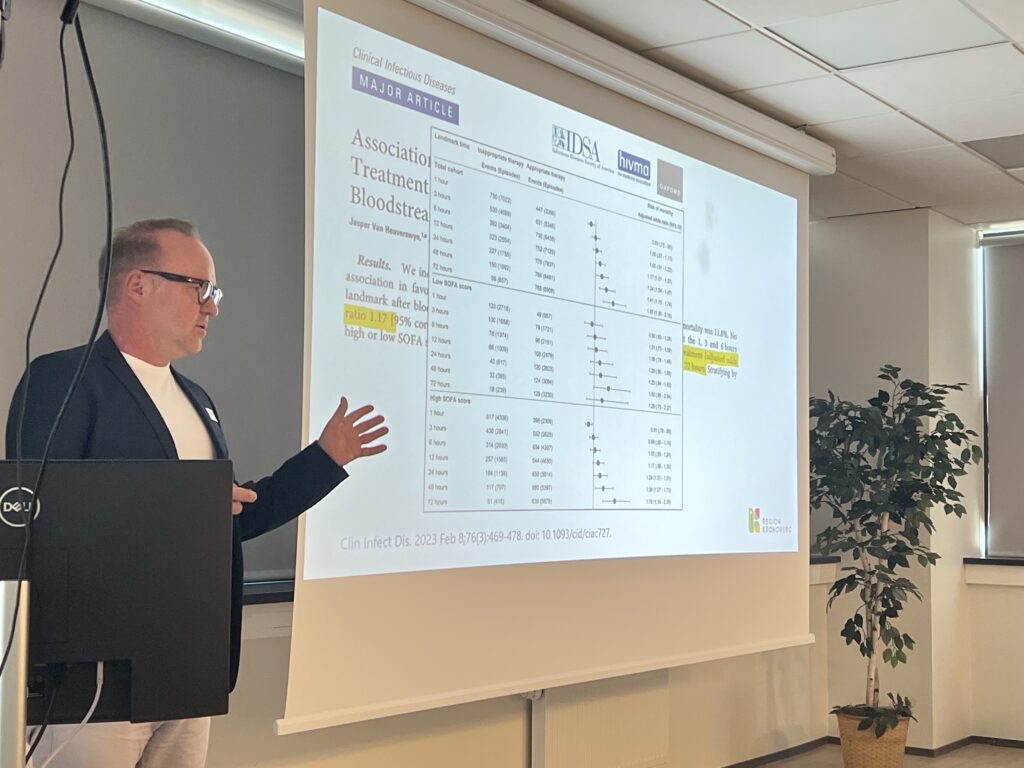
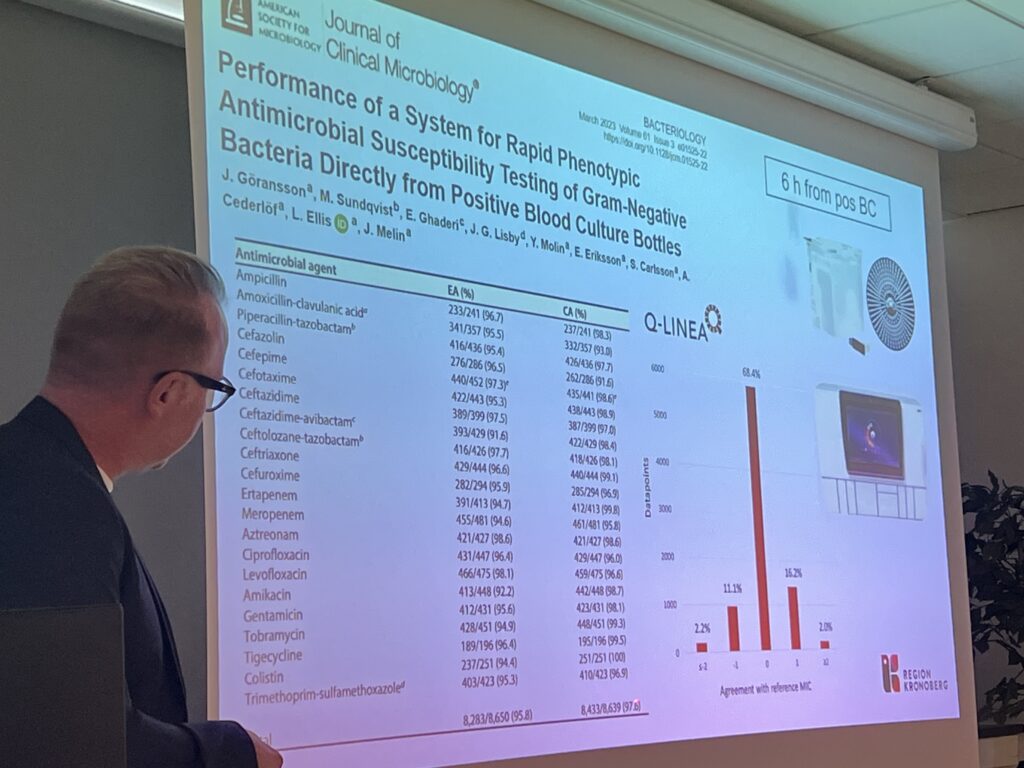
- EUCAST Rapid Antimicrobial Susceptibility Testing (RAST) directly from positive blood cultures
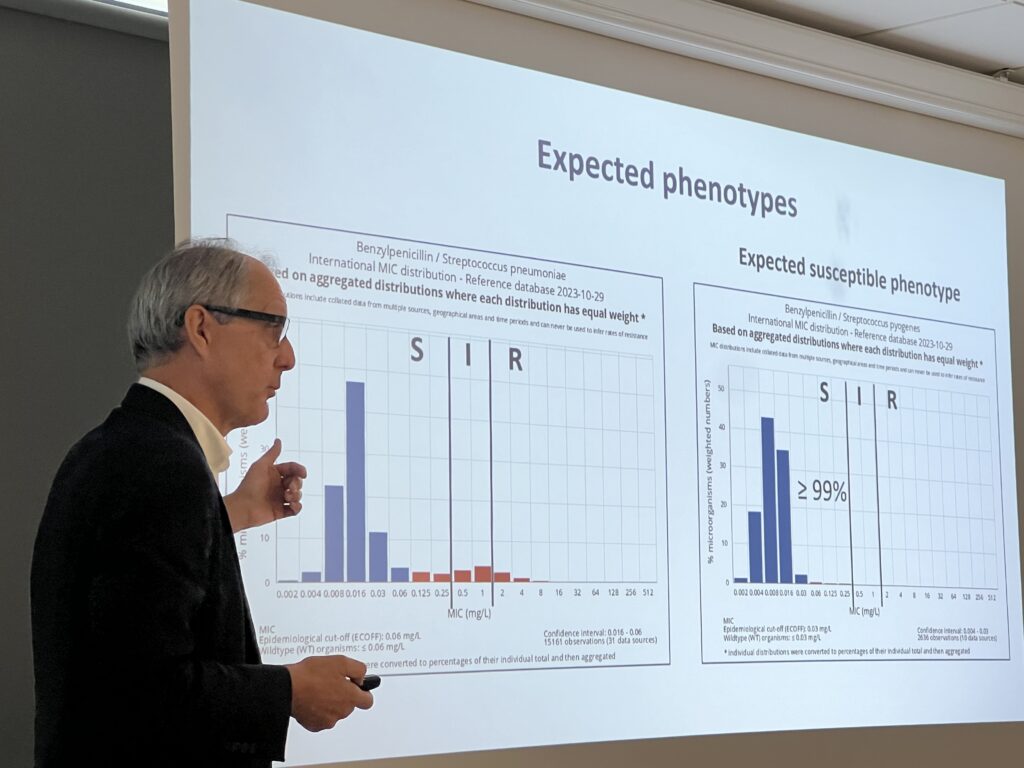
- Expected resistant phenotypes, expected susceptible phenotypes and expert rules
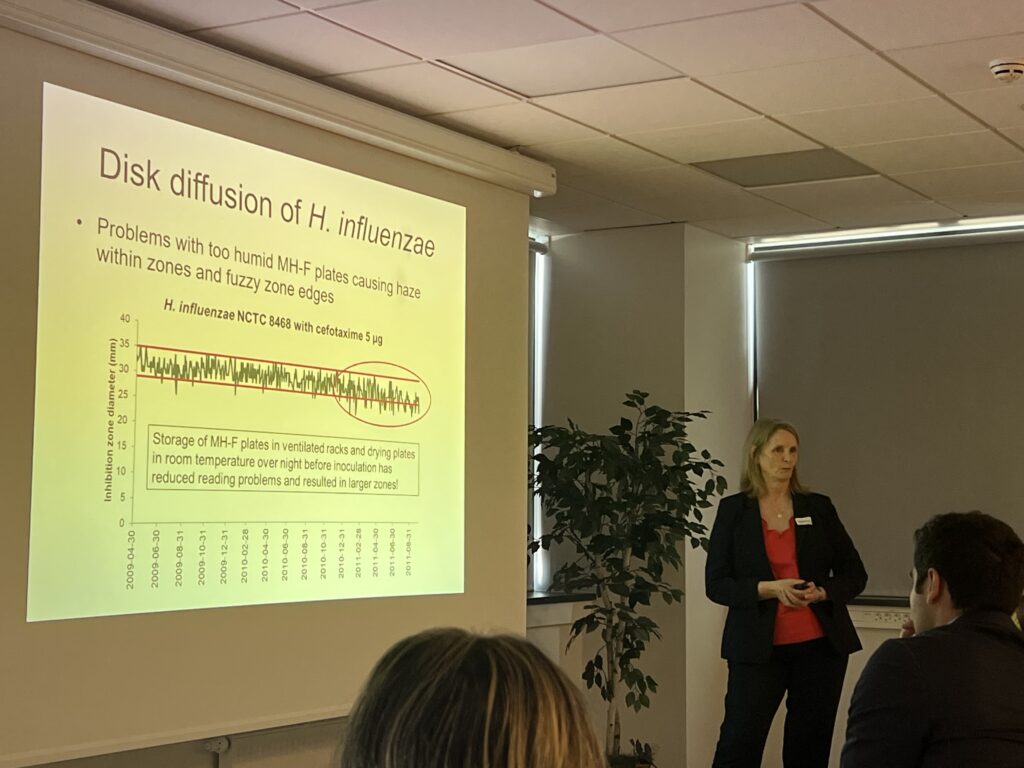
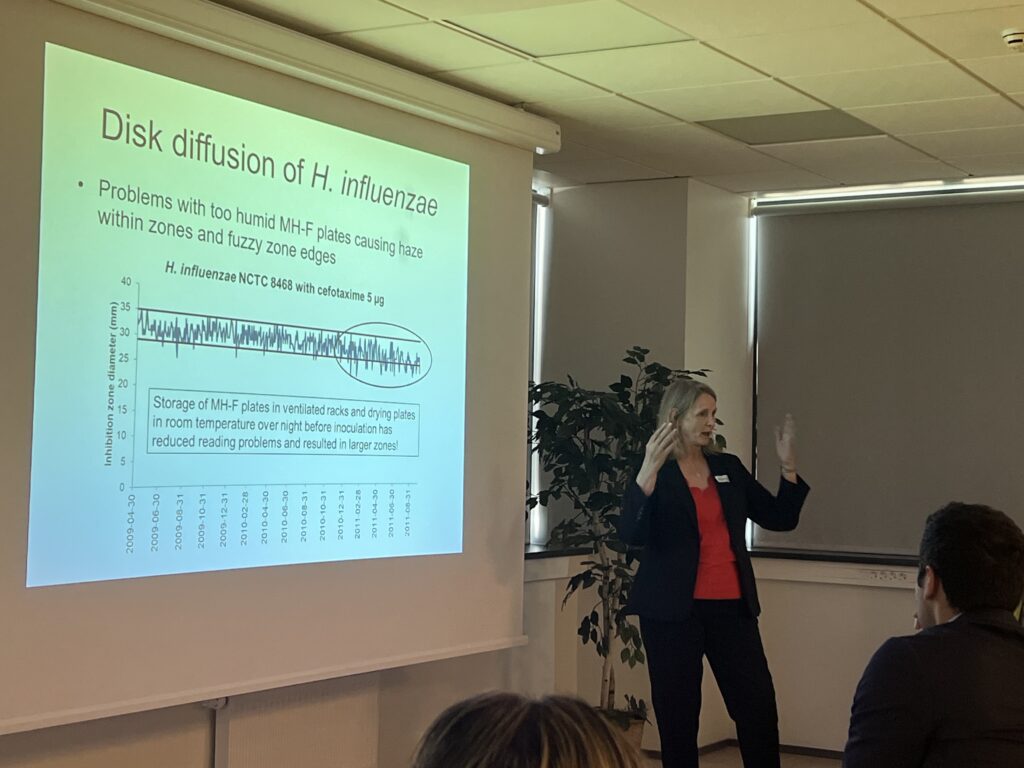
- Quality control in the laboratory
- The role and use of guidance documents, what to do when there are no breakpoints
- Breakpoint tables – How to use and navigate
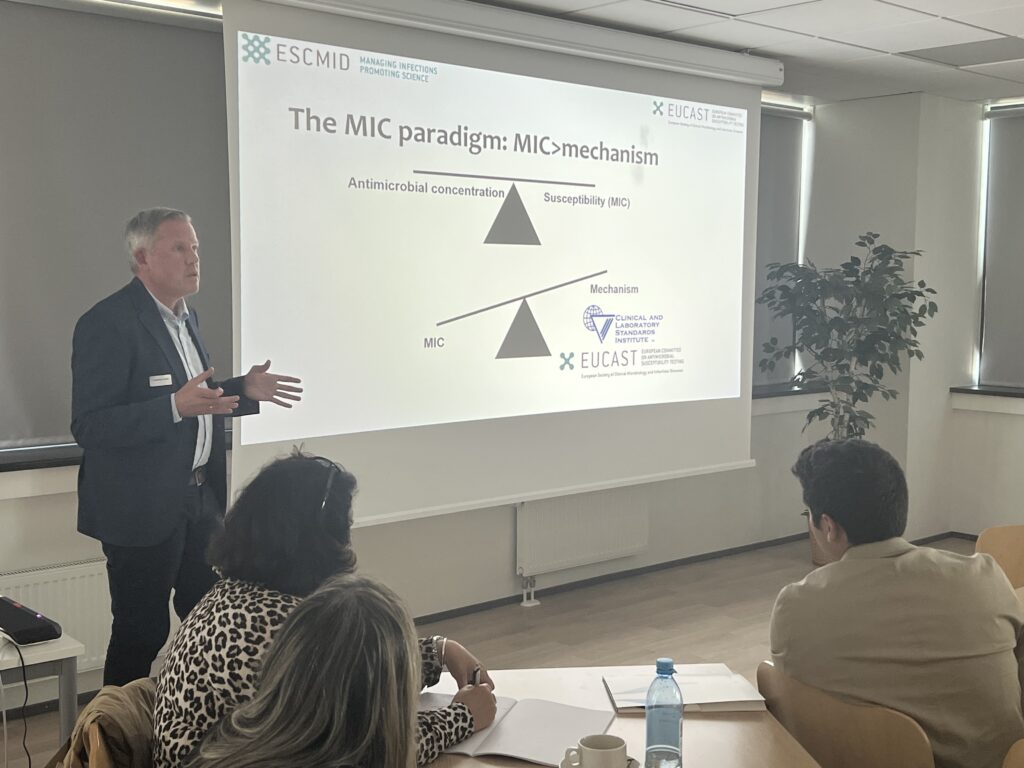
- The EUCAST system, S-I-R definitions
- Requirements for approval of new antibacterials in the EU
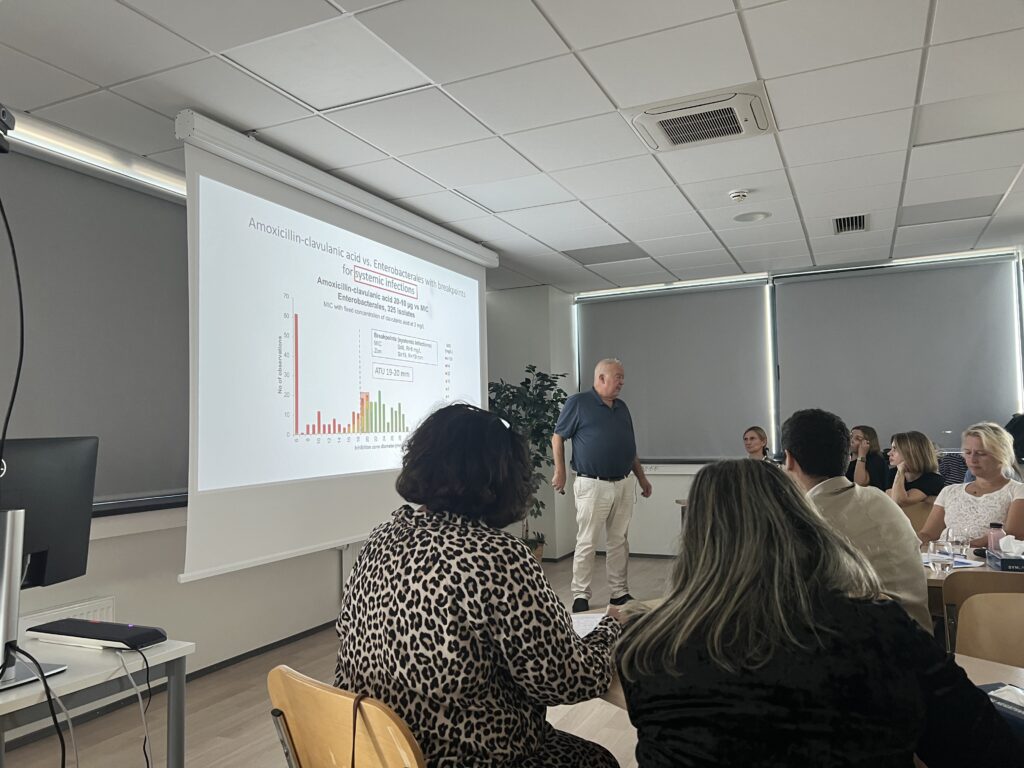
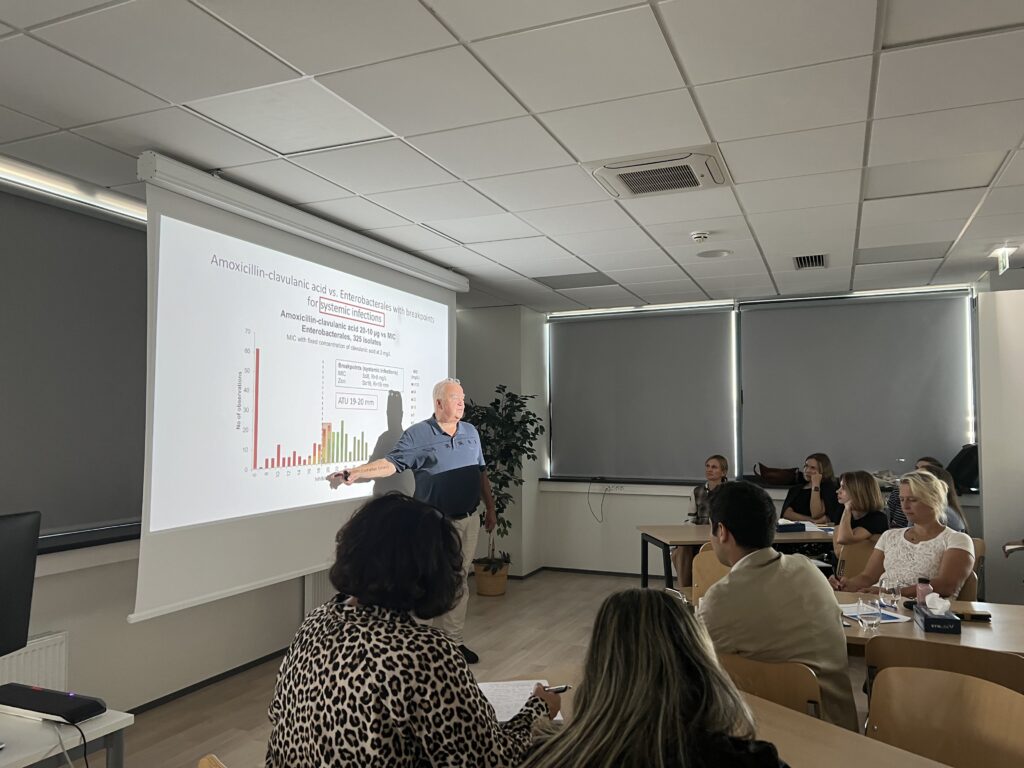
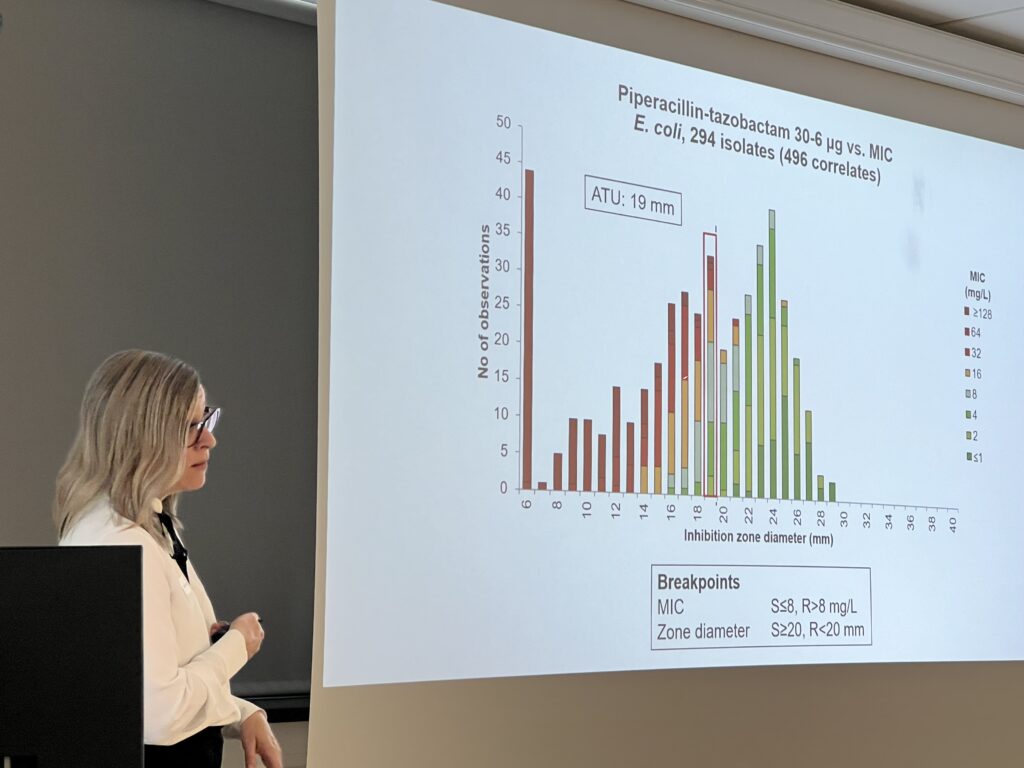
- Wild type MIC (and zone diameter) distributions. ECOFFs (vs. clinical breakpoints)
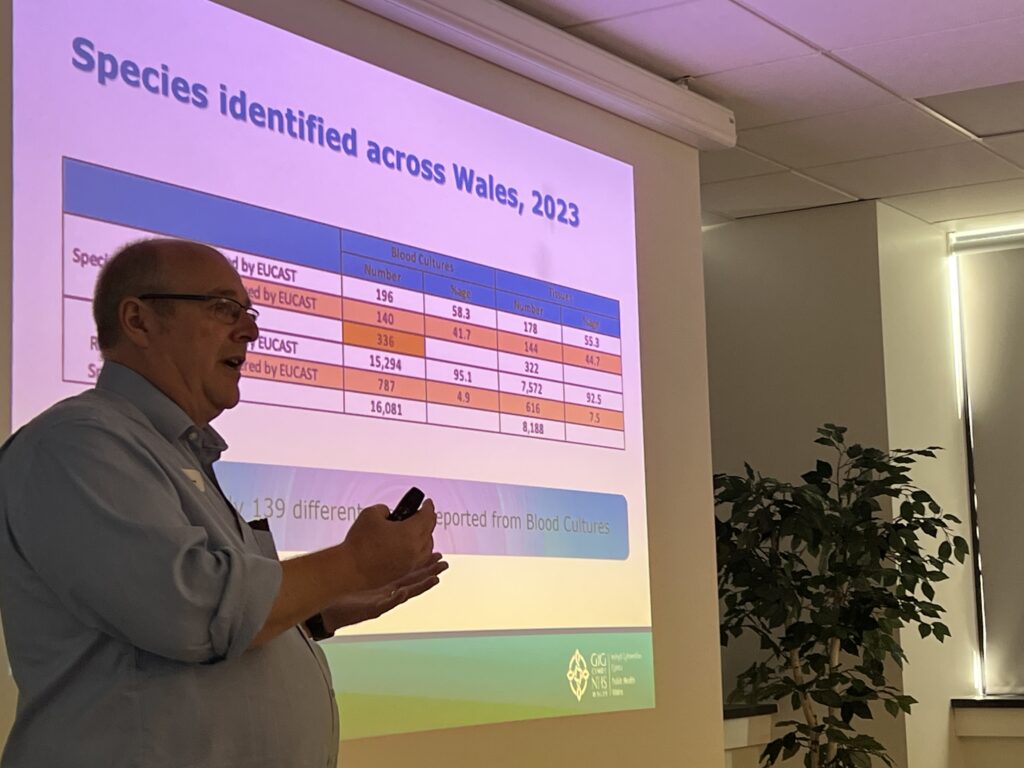
- Susceptibility testing for AMR surveillance – problems and possibilities
- Novel phenotypic methods for AST and detection of resistance
- How clinical breakpoints are set